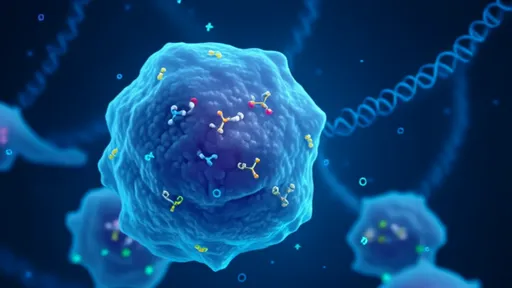
In the ever-evolving landscape of cancer research, scientists are turning their attention to an unconventional form of programmed cell death called pyroptosis. Unlike the more widely studied apoptosis, pyroptosis is characterized by its inflammatory nature – a feature once considered undesirable in cancer therapy but now being reevaluated as a potential weapon against tumors. This fiery cellular demise, marked by cell swelling, membrane rupture, and the release of pro-inflammatory cytokines, is creating waves in oncology circles as researchers uncover its dual role in both promoting and inhibiting cancer progression.
The mechanism of pyroptosis involves the activation of gasdermin proteins, particularly gasdermin D and E, which form pores in the cell membrane upon cleavage by inflammatory caspases or granzymes. These pores allow the release of intracellular contents, including damage-associated molecular patterns (DAMPs) and cytokines like IL-1β and IL-18, which trigger robust immune responses. What makes this pathway particularly intriguing for cancer therapy is its ability to not only kill tumor cells directly but also to stimulate anti-tumor immunity through the release of these inflammatory signals.
Recent studies have revealed that many cancer cells have developed mechanisms to suppress pyroptosis as an evolutionary survival strategy. Tumors often downregulate gasdermin expression or upregulate inhibitory pathways to avoid this inflammatory death. This discovery has opened new avenues for therapeutic intervention, where restoring or inducing pyroptosis in cancer cells could serve as a powerful treatment strategy. Several laboratories have demonstrated that pharmacological induction of pyroptosis can effectively kill treatment-resistant cancer cells while simultaneously activating immune responses against the tumor.
The inflammatory nature of pyroptosis presents both opportunities and challenges. On one hand, the released cytokines can recruit and activate immune cells, creating a more favorable tumor microenvironment for immunotherapy. Clinical observations suggest that tumors with features of pyroptosis often show better responses to immune checkpoint inhibitors. On the other hand, excessive or uncontrolled inflammation could potentially fuel tumor progression in certain contexts or lead to adverse side effects. Researchers are now working to precisely modulate this balance, developing strategies to harness the anti-tumor effects of pyroptosis while minimizing potential pro-tumorigenic consequences.
Several innovative approaches are being explored to target pyroptosis for cancer treatment. These include small molecules that directly activate gasdermin proteins, combination therapies that prime cancer cells for pyroptosis before delivering the lethal trigger, and engineered immune cells designed to induce pyroptosis specifically in tumor cells. Some of these approaches have shown remarkable success in preclinical models, particularly against cancers that are resistant to conventional therapies. The field is now moving rapidly toward clinical translation, with several pyroptosis-targeting agents expected to enter human trials in the coming years.
As with any emerging therapeutic strategy, significant questions remain. Researchers are working to understand why some cancer cells are more susceptible to pyroptosis than others, how to predict which patients will benefit most from pyroptosis-inducing therapies, and how to combine these approaches with existing treatments for maximal effect. The answers to these questions will likely shape the next generation of cancer therapies that harness the power of inflammatory cell death.
The exploration of pyroptosis in cancer therapy represents a paradigm shift in our understanding of cell death pathways and their therapeutic potential. By turning what was once viewed as a dangerous inflammatory process into a controlled anti-tumor weapon, scientists are opening new possibilities for treating even the most aggressive and resistant cancers. As research progresses, pyroptosis modulation may emerge as a cornerstone of combination therapies that simultaneously kill tumor cells and activate the immune system against cancer.
By /Jul 3, 2025
By /Jul 3, 2025
By /Jul 3, 2025
By /Jul 3, 2025

By /Jul 3, 2025
By /Jul 3, 2025
By /Jul 3, 2025

By /Jul 3, 2025

By /Jul 3, 2025

By /Jul 3, 2025

By /Jul 3, 2025

By /Jul 3, 2025

By /Jul 3, 2025

By /Jul 3, 2025

By /Jul 3, 2025

By /Jul 3, 2025
By /Jul 3, 2025
By /Jul 3, 2025

By /Jul 3, 2025
By /Jul 3, 2025