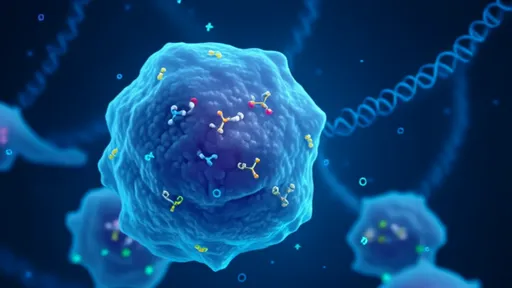
In the intricate symphony of human biology, a groundbreaking discovery has reshaped our understanding of intercellular communication. Extracellular vesicles (EVs), once dismissed as cellular debris, are now recognized as sophisticated nanoscale messengers shuttling critical biological cargo between organs. These tiny membrane-bound particles, ranging from 30 to 1000 nanometers in diameter, carry proteins, lipids, and nucleic acids across vast biological distances, orchestrating physiological harmony and pathological disruptions alike.
The Silent Language of Vesicles
Unlike classical signaling molecules that diffuse passively, EVs operate with remarkable precision. Their lipid bilayer envelopes protect delicate molecular payloads from degradation while allowing targeted delivery to specific cell types. Researchers have observed tumor-derived EVs preparing metastatic niches in distant organs months before cancer cells arrive—a sinister example of biological foreshadowing. Similarly, pancreatic beta cells release insulin-containing EVs that regulate glucose metabolism in the liver, demonstrating their role in maintaining metabolic equilibrium.
What makes this communication system extraordinary is its bidirectional nature. Adipose tissue doesn’t merely store energy; it dispatches hormone-laden EVs that modulate brain appetite centers. Meanwhile, neuronal EVs reciprocate by delivering synaptic proteins to muscle fibers. This constant molecular dialogue occurs at scales where a million EVs could fit on the head of a pin, yet their collective impact governs systemic health.
Breaking Biological Barriers
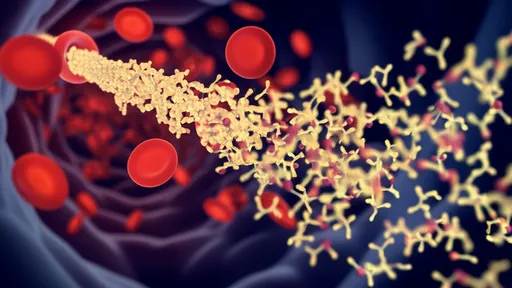
The EV transport system defies conventional biological constraints. While pharmaceutical companies struggle to deliver drugs across the blood-brain barrier, EVs accomplish this feat routinely. Studies show that brain-derived EVs cross into the bloodstream, carrying neurodegenerative disease markers detectable years before clinical symptoms emerge. Conversely, peripheral EVs penetrate the central nervous system, potentially explaining how gut microbiota influence mood disorders through the gut-brain axis.
During pregnancy, a flood of placental EVs reprogram maternal physiology, altering immune tolerance and cardiovascular function. These vesicles act as biological diplomats, negotiating the delicate truce between mother and fetus. Their malfunction correlates with preeclampsia, suggesting that EV-based diagnostics could revolutionize prenatal care. Similarly, exercise-induced EVs mediate the systemic benefits of physical activity, transferring myokines from contracting muscles to distant organs like a molecular fountain of youth.
The Double-Edged Sword of Vesicular Communication
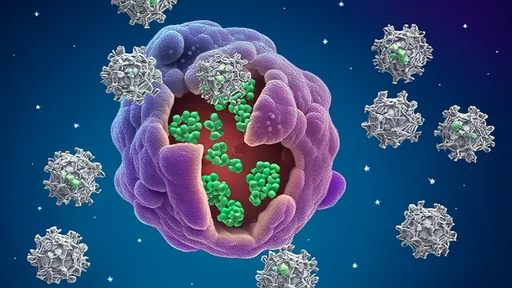
This elegant delivery system has a dark side. Pathogens hijack EV pathways to spread infection—HIV packages viral microRNAs into vesicles that cripple host immunity. Cancer cells weaponize EVs to suppress immune surveillance and prime metastatic sites. In autoimmune diseases like rheumatoid arthritis, pathogenic EVs carry citrullinated peptides that break immune tolerance. Even in metabolic disorders, dysfunctional adipose EVs propagate insulin resistance throughout the body.
Yet these very properties make EVs invaluable therapeutic tools. Engineered EVs loaded with tumor antigens show promise as anticancer vaccines. Mesenchymal stem cell EVs accelerate tissue repair without donor cell risks. The pharmaceutical industry now invests heavily in "EV mimetics"—synthetic nanoparticles replicating natural vesicle functions. From regenerative medicine to targeted drug delivery, bioengineered EVs may soon transform clinical practice.
Decoding the Molecular Postmarks
Scientists are racing to decipher EV addressing systems—the complex surface markers determining their cellular destinations. Some EVs bear integrins homing to specific organs; others display immunomodulatory proteins that dictate immune cell interactions. This "zip code" system explains how bone marrow EVs preferentially migrate to fracture sites or how liver EVs selectively target kidney cells during organ crosstalk.
Advanced technologies now allow single-vesicle analysis, revealing staggering heterogeneity. A single milliliter of blood contains billions of EVs from diverse cellular origins, each with unique molecular signatures. Liquid biopsies exploiting this diversity can detect early-stage cancers or neurodegenerative changes years before conventional imaging. Researchers recently identified Parkinson’s-specific EV signatures in nasal secretions—a finding that could enable noninvasive early diagnosis.
Future Horizons in Vesicular Medicine
The emerging field of vesiculomics aims to catalog EV populations across tissues, developmental stages, and disease states. International consortia are standardizing isolation protocols, addressing current reproducibility challenges. Meanwhile, bioengineers design smart EVs with stimuli-responsive membranes that release cargo only in diseased microenvironments. Some laboratories experiment with EV "postal modifications," chemically altering surface proteins to redirect natural vesicles to therapeutic targets.
As research accelerates, ethical questions surface. Could memory-containing EVs from trained animals enhance cognition in recipients? Should EV-mediated epigenetic information transfer be regulated? These quandaries underscore how profoundly EVs may reshape medicine. From their humble beginnings as cellular dust, extracellular vesicles have risen to become central players in biology’s most sophisticated communication network—a testament to nature’s ingenuity at the nanoscale.
By /Jul 3, 2025
By /Jul 3, 2025
By /Jul 3, 2025
By /Jul 3, 2025

By /Jul 3, 2025
By /Jul 3, 2025
By /Jul 3, 2025

By /Jul 3, 2025

By /Jul 3, 2025

By /Jul 3, 2025

By /Jul 3, 2025

By /Jul 3, 2025

By /Jul 3, 2025

By /Jul 3, 2025

By /Jul 3, 2025

By /Jul 3, 2025
By /Jul 3, 2025
By /Jul 3, 2025

By /Jul 3, 2025
By /Jul 3, 2025